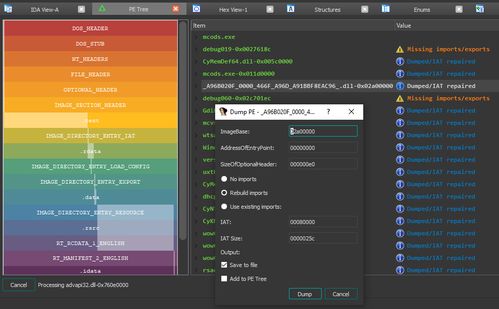
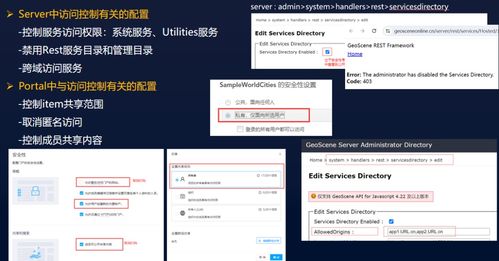
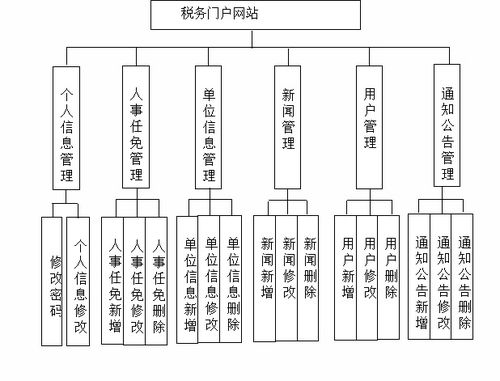
ANSI/CAN/UL 2900-2-1:2018, titled "Standard for Software Cybersecurity for Network-Connectable Products, Part 2-1: Particular Requirements for Network Connectable Components of Healthcare and Wellness Systems," is a critical cybersecurity document developed jointly by Underwriters Laboratories (UL) and the Standards Council of Canada (SCC). This 25-page standard establishes specific software security requirements for network-connectable components within healthcare and wellness systems, addressing the unique risks and regulatory needs of this sensitive sector.
Background and Scope
As part of the broader UL 2900 series, which provides a foundational framework for evaluating the software security of network-connected products, Part 2-1 focuses exclusively on medical and health-related devices and systems. This includes a wide range of components such as patient monitors, infusion pumps, diagnostic imaging equipment, health kiosks, and wellness tracking devices that can connect to a network. The standard is designed to help manufacturers, developers, and integrators identify and mitigate software vulnerabilities that could lead to data breaches, system malfunctions, or threats to patient safety.
Key Requirements and Principles
The standard outlines a comprehensive set of requirements based on established cybersecurity principles. Key areas covered include:
- Secure Development Lifecycle: Mandates processes for secure software design, coding, testing, and maintenance throughout the product's lifecycle.
- Risk Assessment and Management: Requires the identification, evaluation, and mitigation of security risks specific to healthcare environments.
- Software Vulnerability Management: Establishes criteria for identifying, documenting, and remediating known software vulnerabilities.
- Security Controls: Specifies technical controls for access management, data protection (both in transit and at rest), audit logging, and secure software updates.
- Interoperability and System Security: Addresses security considerations for components that must safely interact within larger healthcare IT ecosystems.
The requirements are risk-based, meaning the depth of implementation is scaled according to the potential impact of a security failure on patient health, data confidentiality, and system availability.
Importance for the Healthcare Industry
The healthcare sector is a prime target for cyberattacks due to the high value of personal health information and the critical nature of medical services. A security breach in a medical device can have dire consequences, ranging from theft of sensitive data to direct harm to patients. ANSI/CAN/UL 2900-2-1 provides a standardized, measurable benchmark for security. Compliance helps manufacturers:
- Demonstrate due diligence in product security to healthcare providers, regulators, and patients.
- Align with regulatory expectations from bodies like the U.S. Food and Drug Administration (FDA), which references consensus standards in its pre- and post-market cybersecurity guidance.
- Reduce the risk of costly recalls, liability, and reputational damage associated with security incidents.
- Facilitate safer integration of devices into increasingly connected and interoperable health networks.
Access and Application
The complete 25-page English version of this standard is available as a downloadable resource on platforms like CSDN (China Software Developer Network), categorized under network and information security software development resources. For professionals in medical device software development, health IT, hospital cybersecurity, and regulatory affairs, this document is an essential reference. It serves not only as a compliance checklist but also as a blueprint for building security into the core of healthcare technology products, ultimately contributing to the protection of patient safety and privacy in the digital age.
In summary, ANSI/CAN/UL 2900-2-1:2018 fills a vital niche by translating general cybersecurity principles into actionable, sector-specific requirements for healthcare technology, playing a crucial role in fortifying the digital infrastructure of modern medicine.